A pharmaceutical company came to us with a novel chemical that was FDA and EFSA-approved for oral delivery, but had not yet been evaluated for inhaled delivery. There were several challenges to address, including potential lung toxicity, target specificity, dose optimisation, and regulatory compliance. The company originally intended to use preclinical animal studies to evaluate the safety and efficacy of their new drug. However, ImmuONE were able to provide in vitro human models as a faster, more relevant and accurate alternative.
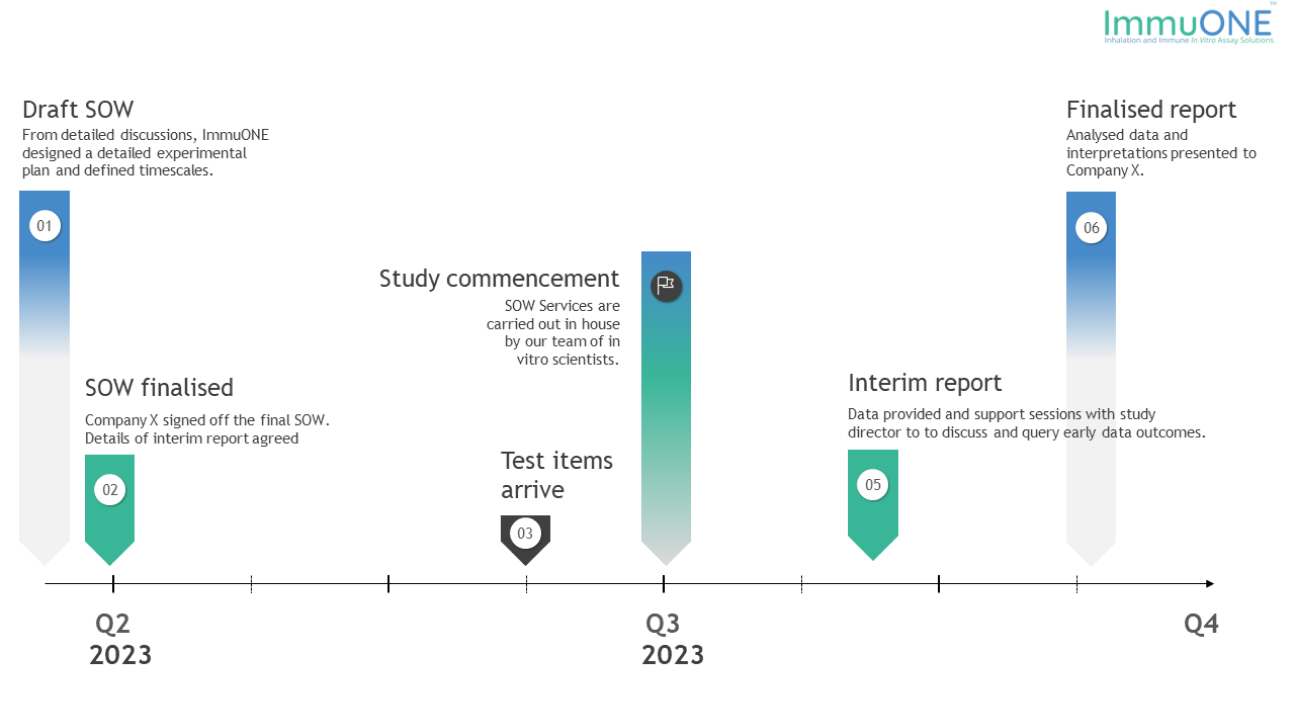
An overview of the company’s timeline with ImmuONE.
What We Proposed
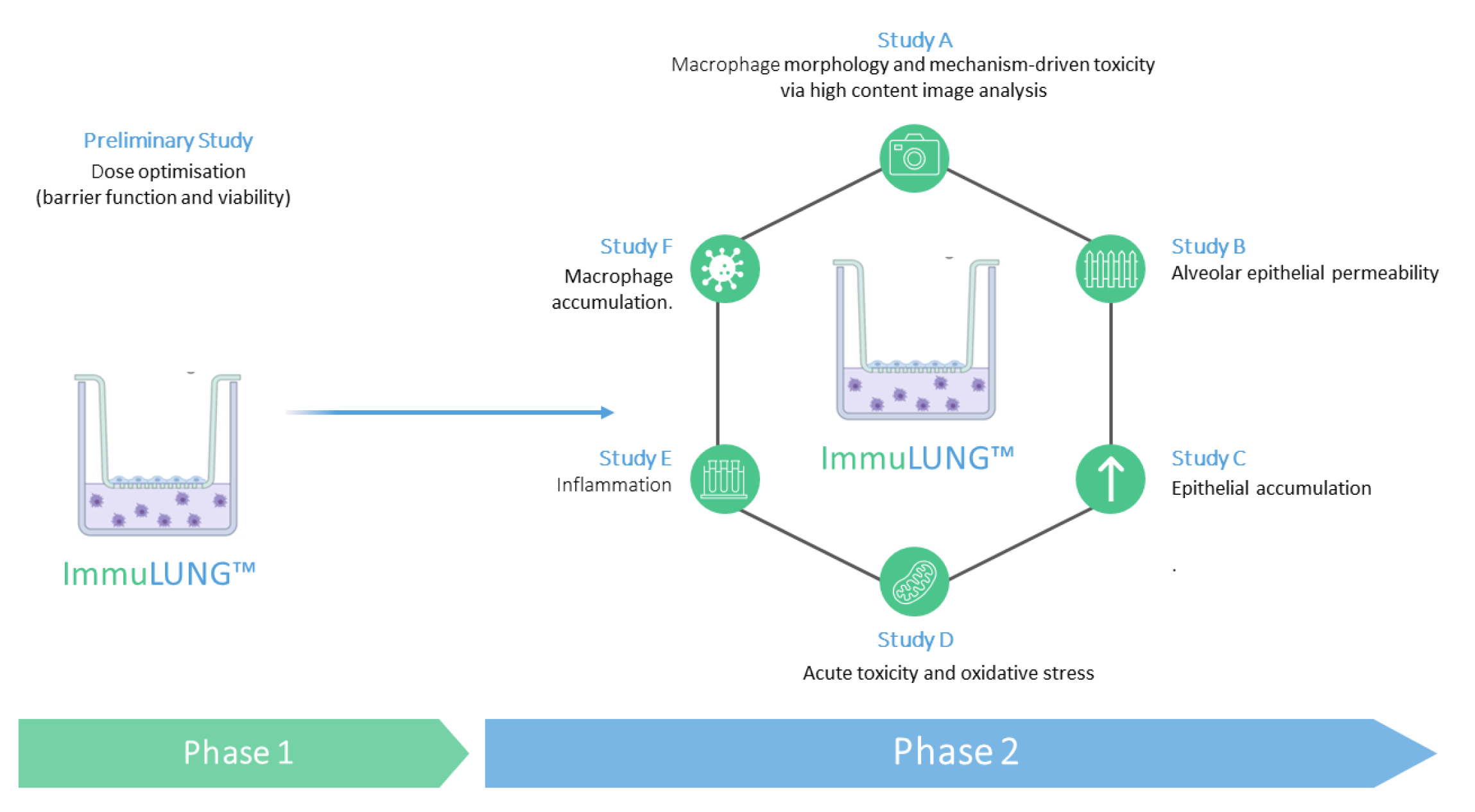
A scope of work proposed by ImmuONE to address the company’s safety concerns.
ABOUT IMMUONE LUNG TOXICITY TESTING
ImmuONE has the expertise and technology to help pharmaceutical companies navigate the rapidly evolving, increasingly complex regulatory landscape. We offer in vitro lung models that are specifically tailored to our clients’ needs. Our innovative in vitro human models are designed to help pharmaceutical companies maintain quality control, develop new products, support marketing claims, and defend against legal claims.
Uniquely, ImmuONE lung toxicity testing for drug discovery:
- Is conducted in a laboratory using human cells, allowing for more human-relevant results
- Can detect even small changes in the immune system to identify chemicals that may harm the lungs, even if they do not cause overt toxicity
- Is efficient, enabling the safe development of new drugs in a timely manner
- is working towards animal-product-free versions to increase human relevance and standardisation