Why macrophages matter in the fight against cystic fibrosis
Lung macrophages play a significant role in the chronic inflammation associated with cystic fibrosis
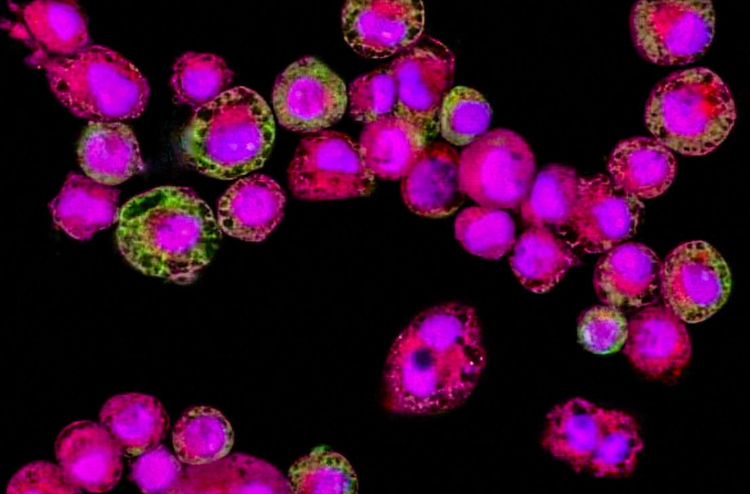
More than half of aerosol medicines under development are terminated due to safety concerns. ImmuONE’s high content imaging or cell painting assays are proven to accurately predict the safety of inhaled compounds by quantifying mechanism-linked morphological and biochemical characteristics within cells, contributing to faster, more informed decision making in the early stages of inhaled drug development.
Recent research by ImmuONE has shown how using high content image analysis of alveolar macrophages can accurately predict the safety of inhaled compounds, which is beneficial for safety testing of compounds in a variety of industries including the pharmaceutical, chemical, agri-chemical, consumer products and cosmetics industries. The technique evaluated in this study forms the basis of ImmuONE’s morph_ONE service, which offers a multi-parametric assessment of the effects of inhaled substances on alveolar macrophage morphology and biochemistry.
Up to 60% of inhaled drug products are withdrawn from development due to safety concerns. This high rate of failure may be partially attributable to the heavy reliance of inhalation toxicity testing on in vivo rodent studies. Immune responses in rat lungs do not correlate well with immune responses in human lungs. Rats significantly differ from humans in lung anatomy, physiology and inflammatory responses.
Alveolar macrophages are the first line of defence against inhaled particulates in the airways. Many candidate inhaled medicines are withdrawn from development due to the induction of abnormal alveolar macrophage morphology in in vivo studies.
The presence of “foamy” macrophages, which are defined by a highly granular or vacuolated cytoplasmic appearance, frequently raises safety concerns. Even though the safety implications of the foamy macrophage response are currently poorly understood, it can result in the failure of drug candidates to progress into clinical development. At present, there is no established assay or biomarker for categorising the foamy macrophage response as adaptive or adverse.
A study published in Toxicology and Applied Pharmacology demonstrates how ImmuONE’s novel multi-parameter HCIA approach can provide human-relevant insights into the safety of inhaled compounds.
Rat and human macrophage cell lines were exposed in vitro to a panel of 15 therapeutic compounds. The panel consisted of inhaled asthma and COPD drugs which are known to be safe, drugs known to induce excess accumulation of phospholipids (phospholipidosis) and drugs known to induce cell death (apoptosis).
High content imaging was then used to characterise cell health, morphology and lipid content. The measurements taken included cell area, nuclear area, cell membrane permeability, mitochondrial activity, phospholipid content per cell and neutral lipid content per cell and drug-induced cell response profiles were generated.
Based on the profiles of both rat and human macrophage cell lines, safe compounds could be distinguished from compounds known to induce apoptosis and phospholipidosis. These results demonstrate that high content imaging approaches are applicable to various in vitro macrophage models and could form a valuable component of inhalation safety assessment. Cell painting assays could help companies make better-informed decisions about whether to advance an inhaled drug or formulation to animal studies, saving time and money.
Rat macrophages were more sensitive to drug exposure than human macrophages, highlighting the need for species-specific testing. Macrophage biology is complex and differs between species on the cellular level.
Based on the drug profiling approach described in this study, ImmuONE is developing a range of high content imaging assays for the inhalation safety assessment of drugs and consumer products. The first of these assays is morph_ONE, which offers insight into how inhaled substances affect more than a dozen morphological features of macrophages.
Using morph_ONE, we can generate phenotypic profiles for new compounds, then predict safety by comparing these compounds to our database of reference compounds with established in vivo responses. The assay uses our human alveolar-like macrophage model ImmuPHAGETM to provide human-relevant insights into drug safety. Results are highly reproducible.
Read the peer-reviewed journal article here.
Connect with us to discuss how our high content imaging or cell painting assays could help you reach your safety assessment goals.

Lung macrophages play a significant role in the chronic inflammation associated with cystic fibrosis

Inhaled substances are primarily tested on rats for toxicity, but key differences between rat and human lungs suggest it’s time to look towards alternative methods.

We’ve just returned from the Society of Toxicology conference in Nashville, where we were excited to exhibit our upcoming in vitro cell culture models.
Make Every Breath Safe
Sycamore House
16 Leyden Road
Stevenage, Hertfordshire, UK
SG1 2BP
Copyright 2025 ImmuONE.
All rights reserved.